Mostly special surface treatments are needed for metal surfaces, especially steels’ surfaces, in order to have the desired optimum properties. There are various techniques for surface treatment but the most common ones are surface coating methods. Steels require further surface coatings for enhanced corrosion resistance, wear resistance, and surface hardness. The surface coating methods differ for different applications. Therefore, the classification of coating techniques can be very complicated. However, the most common surface coating methods can be classified as below:
- Galvanizing
- Electrochemical Coatings
- Vapor Deposition
- Conversion Coatings
- Thermal Spraying
These surface coating methods can be applied for different requirements of applications. Thus these different processes have various advantages and disadvantages.
Galvanizing for Metal Surfaces
Galvanizing is the most common surface coating method which is used almost everywhere in industrial applications. The main mechanism behind galvanizing is the galvanic protection of the base steel. In nature, elements are settled due to their affinity for reduction or oxidation. A determinative array called EMF series is constructed where potential differences between hydrogen and different metals are calculated individually. Therefore, more noble metals are located at the top of the EMF whereas more active metals are located at the bottom. These more active metals tend to corrode more than the noble metals because of their huge potential difference. When two different metals are coupled, the corrosion rate of the more noble one decreases readily where the corrosion rate of the more active one increases. Thus the active metal protects the noble metal from corrosion by lowering its corrosion speed. In the galvanization process, the noble metal is the base steel and the active metal is the coated zinc layer. When the zinc layer gets mechanically injured or scratched, the zinc layer acts as the anode material and the base steel acts as the cathode material so the zinc layer lowers the corrosion rate of the base steel and protects it from corrosion. In the galvanizing process, a multilayer coating occurs. These multilayered form usually consists of intermetallic compounds. The galvanizing process can be mainly separated into two parts which are surface cleaning and zinc coating. At the start of this coating method, surface cleaning is compulsory. Steel coils are transported between long distances and these long distances can be dangerous for steel. Thus coils are greased with different types of oils to protect the surface of the steel during these long transportation intervals. The oil-covered surface of the steel coils must be cleaned with different types of alkali solutions to maintain the perfect removal of the dirt and different type of oils. Proper cleaning ensures good adhesion between the molten zinc and the steel surface. The cleaning part is divided into three sections: Caustic cleaning, pickling, and fluxing. In the caustic cleaning part, the surface of the steel is treated with a hot alkali solution which removes common dirt and oils. In the pickling section, the surface rust and scales are removed by using a hydrochloric acid solution. Finally, in the fluxing part, the surface oxides are removed and protected from further oxidation risks. The cleansed steel sheet is immersed in a zinc bath for coating. The bath composition must contain at least 98% pure molten zinc. The optimum bath chemistry is determined by the ASTM A123 standards. The immersed sheet is removed from the bath when the steel sheet’s temperature approaches a near value to the bath temperature. During the removal of the sheet, the excess zinc is detracted from the sheet by channels that flow pressurized air through the surface of the sheet steel. The desired coating thickness is generally determined by the chemical composition of steel, surface properties of the steel, and cooling rate.
Electrochemical Coatings for Metal Surfaces;
The electrochemical coating method uses the electrostatic properties of different metals which can be either in solution as ions or can be as sheet metals. The electrochemical coatings are divided into two parts; electrodeposition methods and electroless coatings.
- Electrodeposition Method
The electrodeposition surface coating method is a very common technique not for only coating of metal surfaces but for also the extraction of metals. Electrodeposition is also known as electroplating. The technique basically comes from the famous Faraday’s Law. Faraday states that the mass of any substance which is either deposited or liberated from a surface is proportional to its atomic mass and the amount of current passed from the system. Electroplating systems consist of anodes cathodes and electrolytes. A direct current is passed from the system for the liberation or deposition of the ions. The deposition process can be done by using electrorefining processes, electrowinning processes, or molten salt processes. Different metal ions can be coated through the base metal which is used as the cathode in the system. In refining processes, an impure metal is used as an anode and the base metal is used as a cathode. The impure metal anodically polarized by an internal direct current force which causes a liberation of the desired metal ions. Thereafter, the liberated metal ions in the solution are deposited on the base steel which acts as the cathode. The electrowinning process is very similar to the refining process but the only difference is the inert anode material used in the system. The desired ions already located in the solution and there is no need to separate them from a source metal. Ions are again deposited onto the base metals surface which acts as a cathode material. The molten salt technique is unique because some metals can’t be coated by using general refining or winning techniques. The metals located below manganese in EMF series, cannot be coated or produced via using these general techniques. The only electrometallurgical route is the use of a molten salt bath. In molten salt processes, the technique is similar to refining and winning but the bath consists of molten salt composition of the desired coating ion.
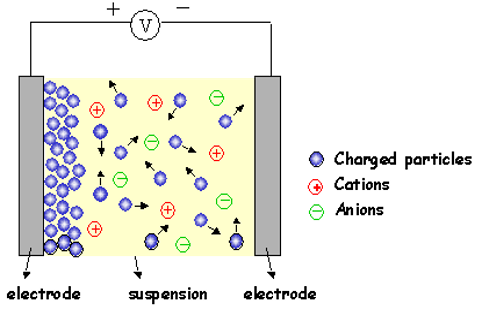
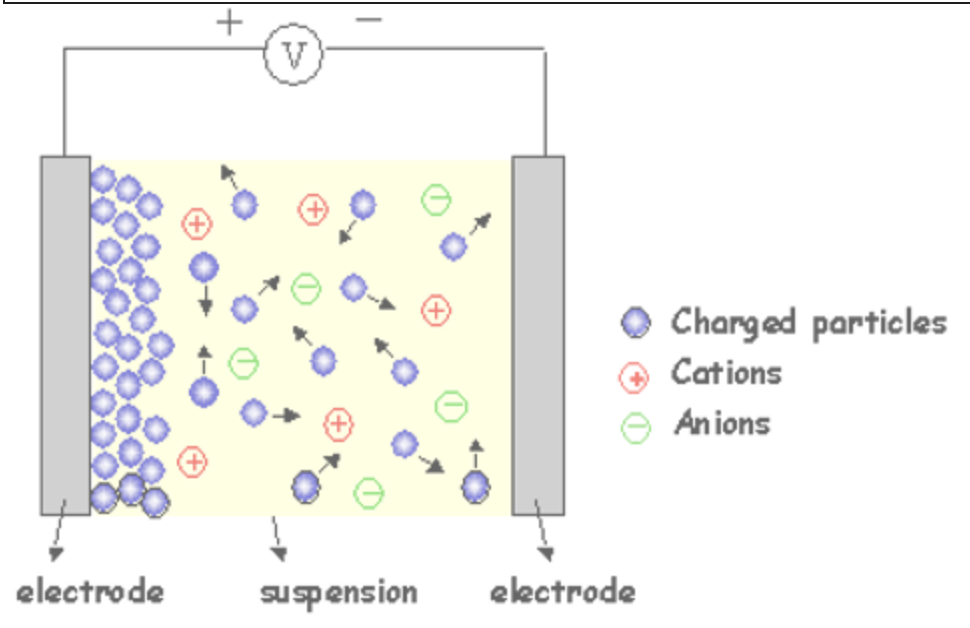
Figure X. Schematic of the electroplating method. Retrieved from: Electrophoretic Deposition (EPD). (2020).28 January 2020, https://www.mtm.kuleuven.be/onderzoek/Ceramics/old-info/EPD
- Electroless Coating Method
The main difference between electroless coating from electroplating is the absence of external electrical power. In this method, the desired surface is first cleaned and immersed in a solution. The surface is etched by the ions in the solution and ions located on the surface. Moreover, the desired coating ion is replaced with the located etchant ions by the difference of potential. Usually, nickel atoms are coated by using this surface coating method.
Vapor Deposition Methods for Metal Surfaces
The vapor deposition methods utilize vaporization and condensation processes for making thin-film layers. The desired metal first evaporated and condensed onto the target surface. Vapor deposition methods divide into two groups; chemical vapor deposition (CVD) and physical vapor deposition (PVD).
- PVD
PVD method can create ultra-thin films from 0-20 micrometers. The ionization or atomization process can be done by physical evaporation of the substance or plasma sputtering. In the physical evaporation method, the metal substance is first evaporated at high temperatures from 1000 to 2000 °C and condensated under a highly pressurized vacuum environment. In the plasma sputtering method, the coating material is emitted from the surface by accelerating different ions to the surface of the substance. Accelerated ions impact the surface of the substance and desired coating atoms are ejected from the surface and deposited onto the desired surface. This process is held under an electrostatic system where the desired substance is used as a cathode. An argon atmosphere is utilized as the medium where argon gas is ionized and ions are accelerated through the desired metal substance and eject them from the surface. The use of the plasma sputtering method enables a high adhesion between the base surface and the coating substance because of the high kinetic energy of the substance ions.
Figure X. A basic representation of PVD technique. Retrieved from: Forschungszentrum Jülich – Employees at IEK-1 – PVD/CVD. (2020). 28 January 2020, https://www.fz-juelich.de/iek/iek-1/EN/Expertise/Duennschichttechnologien/PVD_CVD.html?nn=511174
- CVD
The CVD technique also uses the gaseous phase of the desired metal substance, condensed onto the target base metal surface. In the CVD technique, the substrate is heated at high temperatures over 850 °C. This high-temperature process restricts material selection because only materials with high melting temperatures can be used in this process. First, the precursor substances are introduced into the hot reaction chamber where the target surface is located. The precursors vaporize and are absorbed onto the target surface. Adsorbed substances chemically react with each other and leave behind the desired coating metal on the surface of the target. The desired metal coats an impervious thin layer on the surface of the base metal. This surface coating method is commonly used in the coating of hard materials such as nitrides, carbides, or borides.
Conversion Coatings for Metal Surfaces
Conversion Coatings usually consist of spraying or dipping the desired material through the additional substance. Conversion coatings develop the corrosion resistance of the surface by additional substances such as chromium or phosphate. Moreover, conversion coatings also develop the adhesion properties of the surface for further painting applications. Conversion surface coating methods can be classified as chromating, phosphating, and anodizing. In the chromating process, the base metal is dipped into a solution with chromium ions. Chromate coatings have self-healing properties. The hexavalent chromium leaches slowly when it’s in contact with a liquid or moisture. The dissolved hexavalent chromium ions are adsorbed by the defects and, finally, form a passive film that consists of insoluble trivalent chromium. In the phosphating method, metal surfaces are dipped into the phosphoric acid solution, or solutions are sprayed onto the metal surfaces. A phosphate layer is formed on the surface of the base metal which eases a good adhesion of further painting applications. The anodizing method is different from the chromating and phosphating methods because the protective film is formed by the base metal in the anodizing method. Some metals (e.g. Ti, Al) can form a protective oxide film on their surfaces. When the target metal surface is anodically polarized by an external electrical source, the metal surface starts to corrode and forms a protective oxide film. This impervious layer protects the base metal from further corrosion risks.
Thermal Spraying for Metal Surfaces
In this surface coating method, the substances are heated and are sprayed onto the desired metal surface. The coating thickness can be higher than the other common methods. The heating of the substances is usually done by an external electrical source which can generate an arc or plasma.