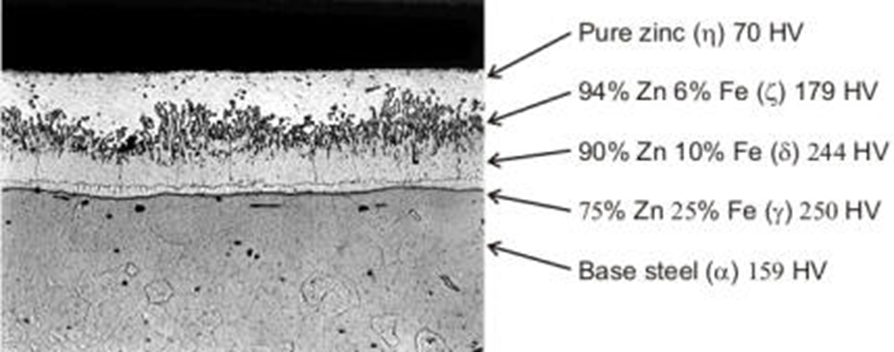
Galvanizing, which is the most rational, economical and definitive solution for the protection of iron and steel against rusting, is the hot dip method of the material, and acts as a barrier against oxidation by bonding with zinc and the iron or steel under it. During galvanizing, the liquid zinc reacts with the iron or steel surface and forms various zinc / iron layers and a protective zinc coating is formed.
https://www.corrosion-doctors.org/Definitions/Galvanizing.htm
There are many factors that can cause problems if not paid attention during the procedure. To give an example to these factors, the composition of the steel, bath temperature, alloying elements in the bath and dipping time are the parameters that should be considered during the process. The composition of the material to be galvanized is the most important parameter. There are types of defects that occur during galvanizing. These defects are explained below on cause-effect relationship in an understandable way and supported by appropriate solution methods[2].
Ungalvanized Weld Areas
Reason: Locally un-galvanized areas around the weld may be due to weld slag, weld pores or weld cavities, and oxide deposits and weld residues are resistant to acid cleaning and must be removed before galvanizing.
Procedures: With mechanical cleaning tools, weld slag residues should be removed by the manufacturer and the residue can be removed by a suitable chipping method or with a brush. Gas metal arc welding is suitable for hot dip galvanizing.
Weld Spatter
Definition: It is the weld metal that is formed as a molten or solid on the material surface during the welding process.
Reason: Occurs due to improper use of welding parameters and explosions in the weld pool when the material is dirty.
Procedures: Weakly adhering weld spatter should be cleaned before hot dip galvanizing, but tightly adhered weld spatter does not affect the wear resistance of the coating, although it is not accepted by the standards[3].
Dark Staining Adjacent to Welds
Reason: Preparation chemicals that act with poor quality welds boil from the connection during galvanizing. As a result, it causes deficiencies in the coating and surface contamination, moreover; Anhydrous flow salts remaining in the connection will absorb atmospheric moisture and will seep into the adjacent galvanized surface.
Procedures: The affected area should be properly washed to eliminate the problem of corrosive leachate. If the salts are leached, equilibrium is reached.
Dull Gray or Mottled Coatings
Definition: It may appear as local matte patches or spread over the entire surface of the part.
Reason: This appearance is due to the presence of intense iron-zinc alloy phase growth caused by phosphorus and silicon.
Procedures: Although matte gray coatings look worse than pure zinc coatings, they provide similar or better protection against wear.
Dross Pimples/Inclusions
Definition: Bubbles formed on the material surface due to surface defects such as dross residues in the hot dip galvanizing.
https://galvanizeit.org/knowledgebase/article/dross-inclusions
Reason: Dros bubbles are formed as a result of material removing the dross layer at the bottom of the furnace or coming into contact with the dross layer and their appearance is in the form of small, hard piles on a normal galvanized surface. Bubbles can also be formed due to hydrogen absorbed during surface cleaning and diffuses at the galvanizing temperature.
Procedures: During galvanizing, contact with the dross layer at the bottom of the furnace should be avoided, and the immersion depth and dross height should be constantly checked. Dros bubbles appear as small bumps on the homogeneous and normal appearance of the coating, so they have no effect on the wear resistance[5].
Ash Staining
Description: Ash that is not cleaned on the surface of liquid zinc before dipping the steel may collect on the surface of the steel during dipping, resulting in uncovered areas under the collected ashes.
Reason: Inadequate cleaning of ash from the surface of liquid zinc before dipping.
Procedures: Small uncoated surfaces must comply with the requirements of certain quality standards while cleaning ash. Large uncoated areas are the reason for rejection and the material must be stripped and re-galvanized.
Deformation
Definition: Deformation is an undesirable shape defect that may occur during the hot dip galvanizing process.
Reason: Hot dip galvanizing is carried out in liquid zinc at 450 ° C, which is the lowest point of the stress relieving temperature for the iron. Therefore, it is possible to eliminate the existing rolling and welding stresses that occur during production, which may cause material changes and deformations.
Procedures: Symmetrical design should be provided, thicknesses of close dimensions should be used, those with non-supported thin wall thickness should be reinforced, pre-shaped parts with the correct minimum bend radius should be used, balanced or wavy zigzag welding technique should be used, temporary support should be used in channels, cylinders and frame angles[5].
Spangled Coatings
Definition: The surface is in silver gray color and generally has a patterned structure (zinc crystals) in different sizes.
Reason: The surface appearance can change according to the chemical composition of the steel and the cooling rate has a direct effect on the surface brightness and scaly structure.
Procedures: Adding a small amount of aluminum (Al) to the molten zinc brightens the coating.
The Effect of Silicon on the Steel to be Galvanized
The reasons affecting the coating thickness and appearance in the galvanizing process are the chemical composition of the steel, the surface roughness of the steel, the processes performed before the steel galvanization, the bath temperature, the immersion time, the speed of removal from the bath, the cooling rate of the steel and minimum values of galvanized coating thicknesses are specified for different material thicknesses in ASTM, EN, ISO, CSA and AASHTO standards. The amounts of the elements in the steel have a significant effect on the coating thickness, the silicon and phosphorus ratios are very effective on both appearance and coating thickness. Silicium, phosphorus or both can create a galvanized appearance in both thick and dark gray colors.
Silicon, which is included in the steel and added to the steel as a deoxidizer; It accelerates the reaction between the steel and the molten zinc. When the material is removed from the galvanization bath, the reaction can continue while it is still hot and it can transform the whole or part of the pure zinc layer on the surface of the part to Zn-Fe alloy. Fe-Zn alloy has a darker gray color compared to bright gray zinc and has a higher abrasion resistance. Generally, Zn-Fe alloyed coatings form thicker coatings and therefore they have a longer service life than coatings on boiling or Al cast steels. Fe-Zn alloys are at least as corrosion resistant as zinc in any case and have better corrosion resistance in acidic industrial environments.
The presence of silicon in the steel to be galvanized causes various difficulties, when all other parameters are constant, it causes the formation of a thicker dull gray surface appearance than normal and a weaker coating adhesion. Taking into account the nature of the material, non-reactive steel would be more appropriate. Although there is no specific criterion in steel selection; carbon ratio less than 0.25%, phosphorus ratio less than 0.04%, manganese ratio less than 1.35%, and the amount of silicon is required to be less than 0.03% or between 0.15% and 0.25%[6].
High reactivity silicon causes a dull gray color and a thicker brittle coating than it should be, and when the silicon on the steel surface is not homogeneously distributed, it causes the coating to be rough, and the reactivity of steel depends on the proportion and distribution of silicon it contains; at the normal 450°C galvanizing temperature, the usual coating takes place, although Si increases reactivity by about 0.03%. In addition, at high Si concentrations such as 3%, the reactivity of the material becomes much less and the iron loss decreases and thin coating occurs. At intermediate concentration values, the reactivity and structure of the layer are quite different, the highest thickness value is in the Sandelin Zone shown in the figure below, and the parabolic curve becomes linear in galvanization times of more than 2 minutes.
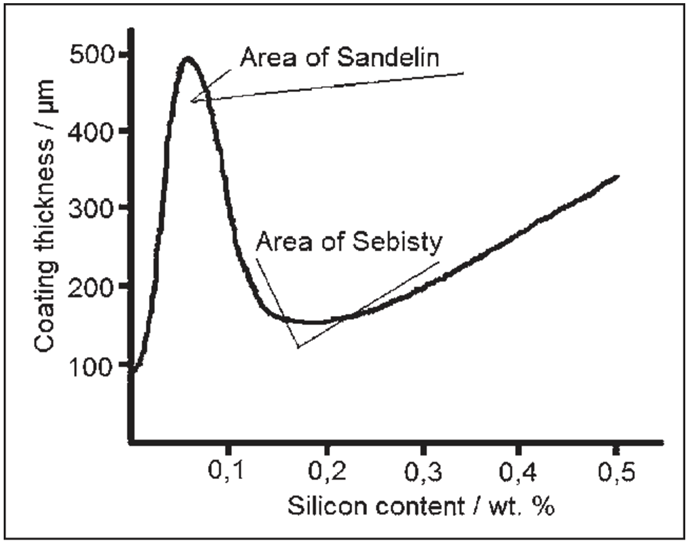
As shown in the figure above, which has been obtained as a result of various experiments, the effect of silicon on galvanization is expressed in terms of the amount of zinc per unit surface in terms of the amount of silicon in the steel, and in order to prevent this formation, the silicon value in the steel must be outside the Sandelin Zone values.
Resources
[1] Retrieved from: https://www.corrosion-doctors.org/Definitions/Galvanizing.htm
[2] “The Hot-Dip Galvanizing Process”. V&S Hot Dip Galvanzing. Archived from the original on 2020-03-18. Retrieved 2012-11-30.
[3] GalvInfo (August 2011). “GalvInfoNote / The Spangle on Hot-Dip Galvanized Steel Sheet” (PDF). GalvInfo. Retrieved 27 February 2014.
[4] https://galvanizeit.org/knowledgebase/article/dross-inclusions
[5] Specification of patent granted to M. Sorel, of Paris, France, December, 1837. Journal of the Franklin Institute (Philadelphia, Pa.), Published by Pergamon Press, 1838.
[6] F. Porter. Zinc Handbook. 1991.
[7] Retriewed from: https://www.semanticscholar.org/paper/Effect-of-chemical-composition-of-steel-on-the-of-–-Pokorný