The advancements in modern technologies make us select better materials for newly developed processes. The enhancements in the applications require better protection for most of the materials. One of the most famous breakthroughs in this manner is the utilization of the galvanized steel. When the topic is galvanized steel, one can remind the enhancement in the corrosion protection performance. Galvanized steel performs increased corrosion protection, which can be considered the uttermost advantage of the galvanization process.
Furthermore, durability against mechanical damages is increased by galvanization. The importance of the galvanized steel can be understood when the market value of galvanized steel is examined. The total worth of the global galvanized steel market is estimated at around 200 billion dollars. In 2025, the projected market value is calculated as 283 billion dollars. An increase above 5% of CAGR (compound annual growth rate) is expected in 2025.
Galvanized steels are commonly used for most production applications which require the utilization of steel as the base material. The versatility of the galvanized steel ensured a broad range of application areas. Galvanized steel products are used from the construction industry to the oil and gas industry. The below schematic shows the well-known application areas of the galvanized steel.
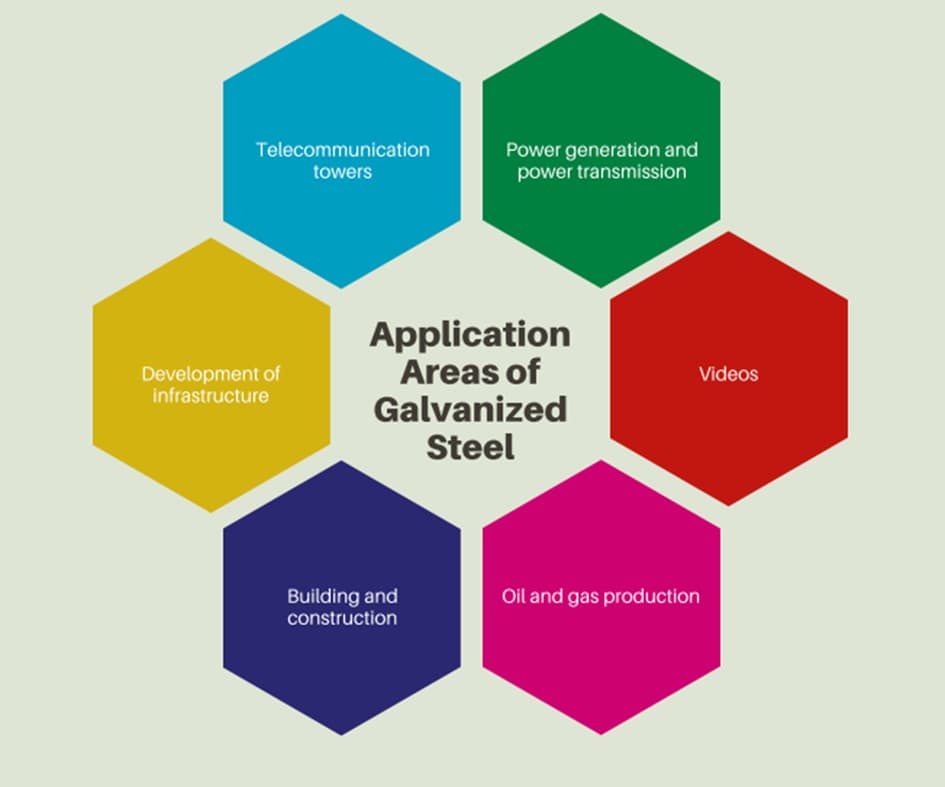
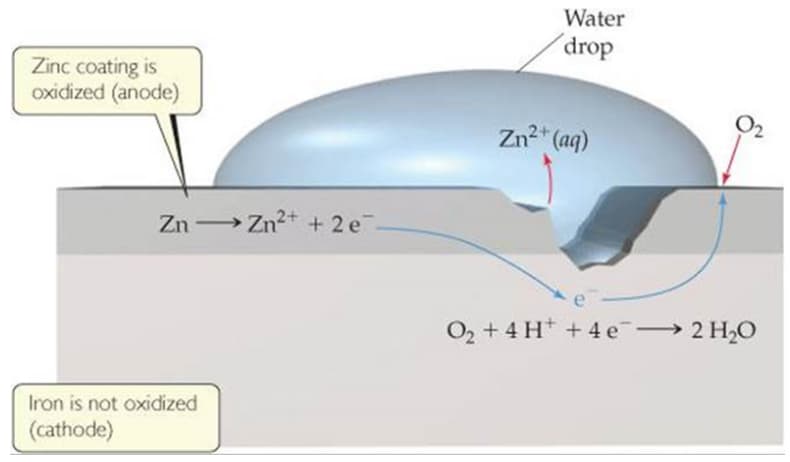
How Is Galvanized Steel Protected From Corrosion Damages?
The basic principle of galvanization is the galvanic protection of the parent steel part. Galvanic steel hinders corrosion progress with the help of the zinc coating. Hence, the passivation of the galvanic steel relies on the electrochemical relationship between steel and zinc. The elements in the world are ordered in an electrochemical array. This electrochemical array is decided by observing the reduction and oxidation abilities of the elements. In other words, the affinity of the elements against reduction and oxidation determines these elements’ positions in order.
The reactivity array of the elements against oxidation is called the EMF series. In the EMF series, each element’s potential difference against hydrogen is calculated, and these series are constructed. EMF series represents more noble metals at the top side and more active metals at the bottom side. In the middle of the series, half-noble elements are located (such as copper). The below figure represents the EMF series.

The elements at the down of the series are more active than those at the top, which means bottom-sided elements are more likely to corrode when compared with the top-sided ones. When a more noble metal is coupled with a more active metal, in other words, if one couples a top-sided element with a bottom-sided element, the corrosion rate of each element changes. The alteration of the corrosion rate causes adverse effects on noble and reactive elements. At the end of the coupling, the corrosion rate of the noble metal decreases, whereas the corrosion rate of active metal increases. Hence, when noble and active metals are coupled, noble metal becomes less likely to corrode. However, corrosion emerges on the active metal faster than the standard conditions (standard conditions means the array in the EMF series)
The fundamentals of galvanization can be explained by considering the EMF series and coupling conditions. Iron (actually, it’s steel, but as you know, steel is an iron-carbon alloy which at least contains 98% iron) is covered with zinc to hinder the possible corrosion damages. When iron is coated with zinc, iron and zinc are also coupled. Iron can be found at higher places than the zinc in the EMF series, so one can easily say that iron is nobler than zinc. Thus, coupling iron and zinc reduces the corrosion rate of iron (steel). Galvanized steel is protected from corrosion by lowering the corrosion rate itself. In brief, the zinc layer lowers the corrosion rate of steel and protects it. That’s why galvanized steel performs excellent performance in corrosive environments.
What Is The Process Route Of Galvanized Steel?
The utilization areas of the galvanized steel are mentioned before, so one can understand that the proper processing of the galvanized steel is vital for further usage. Galvanized steel processing can be introduced in two fundamental parts, which are cleaning of the steel surfaces and coating of molten zinc. The cleansing step of the process is crucial to ensure the adequate coating of zinc on the steel surfaces. The metal sheets and coils are protected against corrosion by greasing. Hence, the oil layer on metals must be removed before the galvanization. To conduct the removal phase, several types of alkali solutions are used. Moreover, excess dirt on the surface is removed, too. The proper cleaning of the surfaces provides better adhesion of the molten zinc. The cleansing part can be separated into three sections, which are caustic cleaning, pickling, and fluxing.
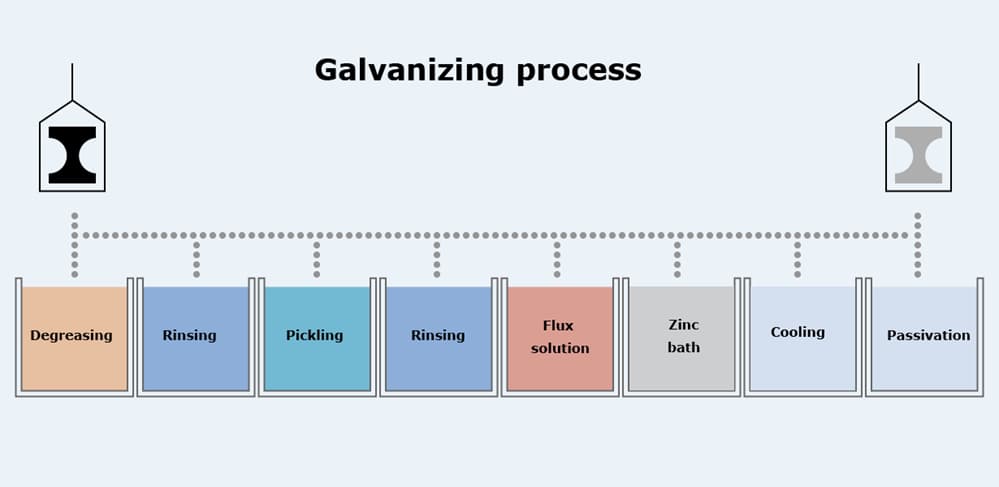
In the hot-dip galvanizing process, cleaned steel parts are immersed into the molten zinc bath. The minimum zinc composition in the bath must be controlled, so at least 98% of the bath must preserve pure zinc. The composition variation of the zinc bath is determined according to the ASTM A123 standards. When the temperature of the steel is reached to the temperature of the bath (470-500 °), immersed steel sheets are taken out of the bath. The thickness of the zinc coating is determined by varying parameters. Hence, the desired thickness of the layer is provided by flowing pressurized air through the steel surface. The pressurized air ensures an equal coating thickness at every point of the steel surface.
Furthermore, steel parts are passivated by implementing chromic acid through the surfaces. Finally, galvanized steel sheets are painted and sheared in accordance with the client’s desires. The processing route of galvanized steel is not that complicated; however, every single step of the process requires uttermost attention.
What Are The Benefits Of Galvanized Steel?
The wide range of application areas of galvanized steel can be counted as a piece of evidence for the benefits of the galvanized steel. The most popular benefits of galvanized steel can be exemplified as excellent corrosion protection, ease of surface modification, and excellent protection against mechanical injuries. Moreover, the cost-efficient features of the galvanizing process make them desirable for most vendors. The need for the galvanized steel for periodic maintenance is lesser than ordinary carbon steels, thanks to the zinc coating. Hence, long time cost efficiency of galvanized steel may be counted as a massive factor for material selection. The below diagram is constructed to show the essential advantages of galvanized steel. As mentioned before, the protective feature of the zinc coating and process quickness can be thought of as the major advantages of the galvanization process. The tough layer on the galvanized steel performs better resistivity against scratches and damages.
When the benefits of the galvanized steel are listed, some significant contributions of the zinc coating may be specified as below articles;
- Lower first cost: When compared with the other surface coating processes, the cost-efficiency rate of the galvanizing process is notably higher than most of the coating operations. The cost-effective feature of galvanized steel made it a preferred material for engineering applications
- Long life: The estimated lifetime of galvanized steel is determined as 50 years for rustic environments. Moreover, even in corrosive environments, galvanized steel can serve for 20 to 25 years. Thus, the long service life of galvanized steel generates a considerable advantage for most the industries
- Tough coating: The iron-zinc multilayer of galvanized steel increases the strength of the steel surface. Therof, zinc coating develops the mechanical properties of the galvanized steel.

- Total coating: The immersion process during the production of galvanized steel provides a full coating at every point on the steel’s surface. Hence, a continuous layer on the steel surface can be acquired. Even in hidden sections or in sharp corners, galvanization can be done entirely. This complete coating of the galvanized steel performs homogenous protection.
- Shorter processing duration: Galvanized steel can be produced from raw material to end material in a couple of minutes. The quickness of the galvanized steel processing makes numerous products available in short times.
Other article that may interest: What Defects Are Commonly Observed During Galvanization?


