Hot dip galvanizing can be described as the most common surface coating method for industrial steel sections. The popularity of hot dip galvanizing comes from its simplicity and efficiency. Hot dip galvanizing is not solely used for corrosion protection; the process also offers increased mechanical properties for candidate metal surfaces. Especially, the scratch resistance of metal surfaces substantially improves when hot dip galvanizing is applied to those surfaces. To attain better wear properties and corrosion protection, hot dip galvanizing can be considered the most famous and efficient process.

When the historical evolution of hot dip galvanizing is examined, the initiation of the process was triggered by french chemist Melouin in 1741. Melouin found out that zinc can be used for the protection of steel from the outer corrosive environment. However, industrial utilization of hot dip galvanizing is started in the early 19th.
Fundamentals of the hot dip galvanizing are based on the so-called ‘galvanic cells’. That is why the process of zinc coating is called galvanizing. Hot dip galvanizing ensures corrosion protection of the substrate by the zinc layer. If the galvanized surface is mechanically injured vaguely or seriously, steel in the damage zone acts as a cathode and protects itself from corrosion. Galvanizing the steel surfaces can be maintained in several ways; however, the most common method is hot dip galvanizing. The hot dip galvanizing process has some advantages, whereas; disadvantages of the process must be mentioned. The below chart is illustrated to represent the pros and cons of the hot dip galvanizing process.

1) Hot Dip Galvanizing Methods
Hot dip galvanizing can be processed via two fundamental methods which are dry galvanizing and wet galvanizing. The difference between the methods comes from the differentiation of fluxing steps for each process. Nonetheless, the initiation and progress of both methods in very similar to each other.
At the beginning of the galvanization process, steel surfaces are cleaned using mechanical methods such as grinding or blasting. Since some marking or painting traces still remain on the surface of the steel, removal of these contaminants must be done.
Steel pieces, especially steel coils, are transported overseas, so protection of these parts is vital. Hence, a proper preparation for hot dip galvanizing, surface cleansing of these steel parts is ensured. During transportation, steel surfaces are covered with oil or grease to hinder the adverse effects of salinary environments. These oil and grease layer is removed in an alkaline solution. Moreover, steel surfaces are washed in hot water and processes in diluted hydrochloric acid (or sulphuric acid). This is called pickling. The pickling step is implemented for removing the mill scales and rusty regions.
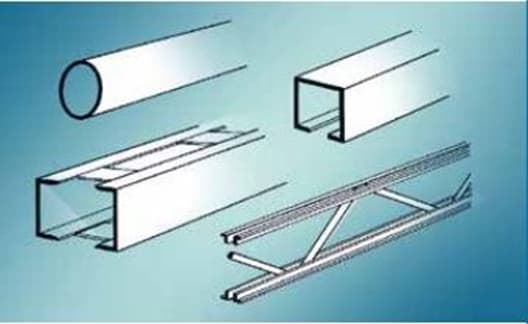
After the pickling step, steel profiles are immersed into the molten zinc bath. During the dipping process, utilization of the fluxing agent is a necessity for most of the opreations. The fluxing addition aims to dissolve oxide scales on both steel surfaces and molten zinc. The dissolution of oxide compounds is vital to yield a superior contact between iron and zinc. As mentioned before, hot dip galvanizing can be separated into two groups. Here, the addition of the fluxing agent selects the type of hot dip galvanizing. In brief, the fluxing agent can be introduced by implementing either dry galvanizing or wet galvanizing methods.
As a method of hot dip galvanizing, dry galvanizing comprises degreasing pickling and rinsing methods. When these steps are completed, steel parts are immersed in a fluxing solution. The fluxing solution contains zinc-ammonium chloride. After immersing through the fluxing bath, surfaces are dried. Consequently, steel surfaces are dipped into a molten zinc bath where hot dip galvanizing is maintained. When the components completely in the zinc bath, fluxing residues or oxide compounds are skimmed from the surface of the zinc bath. The residues are sourced from the fluxing operation, where a thin layer of fluxing salts forms on the surface of the immersed steel sections. When the steel pieces are taken out from the molten zinc bath, sections are cleansed and cooled underwater.
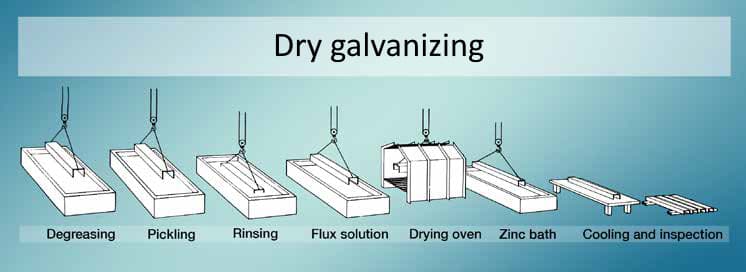
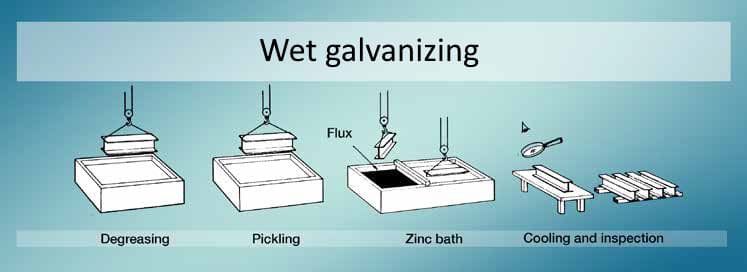
Another method of hot dip galvanizing is described as the wet galvanizing process. In this method, the molten zinc bath is separated into two sections by using a beam. In the first section of the bath, the fluxing agent is deposited (ammonium chloride is widely used as the flux agent). After the pickling step of the hot dip galvanizing process, steel components are immersed in the first section of the bath, where molten zinc contains flux agent. Thereafter, steel components are taken from the first section of the bath and dipped into the second section. The second section of the bath preserves only pure molten zinc. Flux residues and oxide compounds are skimmed here from the surface of the bath. As in the dry galvanizing method, steel sections are cooled in water media.
When two methods are examined separately; both wet galvanizing and dry galvanizing performs substantial quality and corrosion protection. Since the aim of hot dip galvanizing is corrosion protection of steel surfaces, both methods can be utilized for most of the processes. However, industrial applications prefer dry galvanizing as the hot dip galvanizing method. The reason for selecting the dry galvanizing method can be explained when time and money are taken into account. Most vendors prefer dry galvanization because of its simplicity.
2) Iron and Zinc Reactions
The hot dip galvanizing process is utilized for covering a thin zinc layer on the steel substrate. Hence, zinc coating results in the formation of intermetallic compounds between iron and zinc metals. Since the temperature of the process is slightly high, the diffusion of zinc atoms through the surface of the steel substrate increases. An increment in the distribution of the particles leads to the formation of new compounds between the iron-zinc boundary. The standard temperature of hot dip galvanizing is 460 °C, where pure zinc melts. Nevertheless, for small parts, this bath temperature increases up to 550-575 °C.
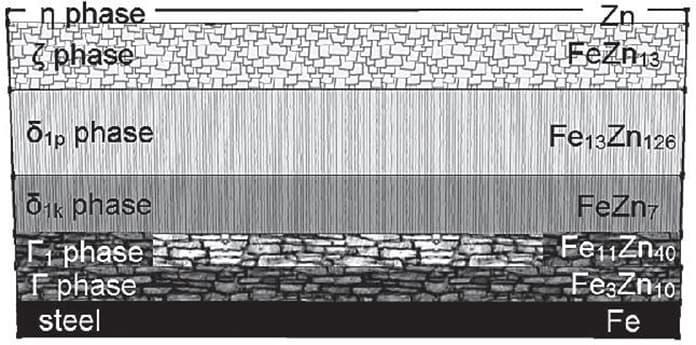
The interaction between iron and zinc generates different iron-zinc layers. The iron and zinc composition changes from layer to layer. A general investigation of the iron zinc layer reveals that the iron content decreases through the outwards of the surface. The outermost layer resulted in the hot dip galvanizing contains only pure zinc. The reason for this pure zinc composition of the outermost zinc layer can be explained with the fundamentals of hot dip galvanizing. When the steel pieces are taken out from the molten zinc bath, a thin pure zinc layer adheres to the generated iron-zinc intermetallics. The below schematic represents the iron- zinc compounds that are generated during the hot dip galvanizing processes. Note that, as the thickness and type of the layers change, the steel surface’s mechanical properties also change. These may affect fatigue properties of the steel parts, hence one may consider this phenomenon if the steel part is designed for cyclic motions.
3) Strength of Hot Dip Galvanized Sections
Numerous experiments have been conducted to test the effect of hot dip galvanizing on steel components over the years. The most famous strength indicators, yield, and tensile points, and fracture strength, were examined to attain consistent results. Yield strength, tensile strength, and rupture elongation values have remained the same with mild carbon steel pieces. However, a slight reduction in fracture toughness was detected on galvanized steel samples.
4) Corrosion of Zinc Layers Under Liquid Medium
Hot dip galvanizing is utilized for enhancing the corrosion resistance of steel parts. Hence, the corrosion performance of coated parts is the preliminary subject of the hot dip galvanizing process. When a zinc coated steel component is immersed into a liquid environment, the steel part is covered with protective corrosion products (mostly impervious oxide scales). Hence the resultant corrosion products slower the corrosion rate. However, some alkaline or acidic liquids may remove the resultant layer, which causes an acceleration in the corrosion rate.
5) Arc Welding of Galvanized Parts
Welding performance or weldability of hot dip galvanizing products shows similarities with plain carbon steels. Actually, there is no significant difference between zinc coated and plain carbon steels in the manner of weldability. However, welding parameters of plain carbon steel cannot be directly used for coated steel parts because of the zinc layer. Here, essential parameters can be exemplified as zinc layer’s thickness, zinc layer’s composition, and structure. Thus, differentiation of welding performance can be solely mentioned about welding data.
Other article that may interest: What Defects Are Commonly Observed During Galvanization?