Steels are produced by alloying iron with carbon. Recycling from iron ore or scrap can be used for steelmaking. The liquid steel produced is shaped as ingot by casting or as a billet or bloom by continuous casting method. Alloying elements have very critical role on steel properties. Especially in the last 2 centuries, humanity has benefited from steel and alloying elements very well. We can examine this alloying process from the iron-carbon balanced diagram. Iron and carbon alloys can be classified into steel and cast iron. We name alloys formed up to 2.14% carbon content in iron as steel. From this composition to the carbon composition of 6.7% by weight, it is cast iron. A eutectic transformation occurs at 727 ̊ C degrees containing 0.76% carbon composition by weight.
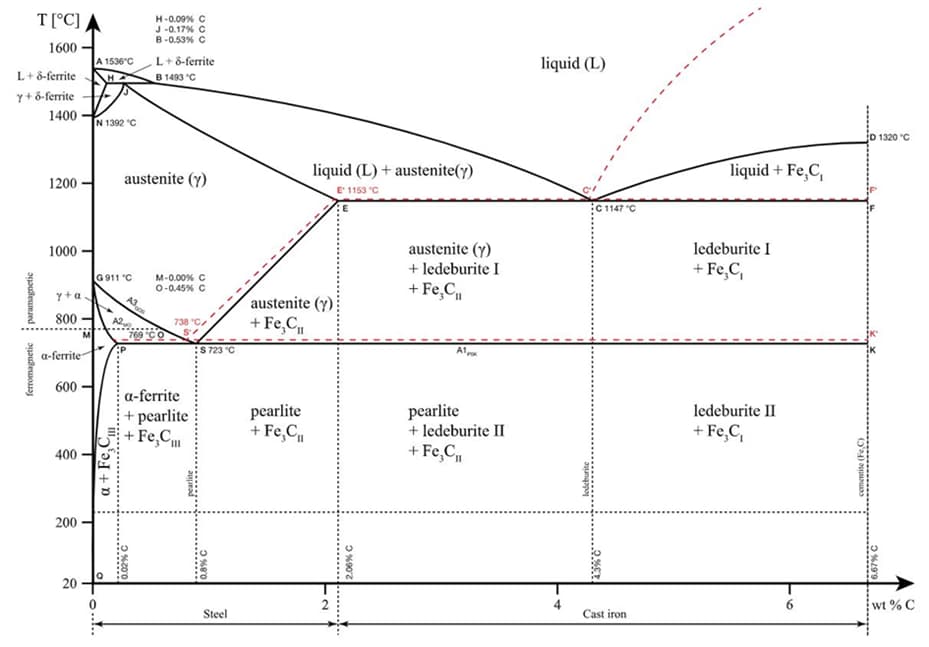
Carbon is the main alloying element of steel. Some impurities may be formed during alloying and it may contain small amounts of other elements as a result of external factors. We can classify steels in 3 different ways according to their composition:
1. Carbon steels;
- Low carbon steels (C <0.25%)
- Medium carbon steels (0.25% <C <0.55%)
- High carbon steels (0.55% <C)
2. Alloy steels;
- Low alloy steels
Low alloy steels contain less than 5% alloying elements in their composition.
- High alloyed steels
High alloy steels contain more than 5% alloying elements in their composition.
3. Steels according to the alloying element in their composition;
- Stainless steels
- Manganese steels
- Chrome-nickel steels
Steel; It is used in many different fields such as kitchen appliances, household appliances, automotive, petroleum industry, leather industry, chemical industry, pump and compressor parts, aviation, nuclear industry. Some changes are made in the features according to the usage areas. The properties of steel vary depending on the carbon composition and alloying elements it contains. Each alloying element adds different properties to steel at different rates. Manufacturers, on the other hand, produce materials that meet the expectations by adjusting these compositions according to the expectations in the area of use of the material they produce. Expecting a material to give all the properties in the best way is both very difficult and very costly. Therefore, it is very important to determine the area of use and which features are most important during this use.
For example, when designing a faucet for a home user, this material will frequently come into contact with water. For this reason, the risk of corrosion is high. The corrosion resistance of the material should be kept high. It will not carry any spares on the material. For this reason, very large compression strength is not required.
Alloying Elements and How They Effect the Steel

Carbon, the main alloying element of steel; Increases mechanical properties such as strength, hardness, and mechanical resistance. But besides this increase, malleability, ductility, and toughness decrease. In addition, the tensile strength can increase up to a point. The increase in the carbon content in the steel composition reduces the ductility of the material, that is, it causes it to show brittle properties. There is a risk of cracking in high carbon steels due to residual austenite that will occur after the heat treatment is applied. It negatively affects the forging and weldability properties of steel.
Chromium is the most commonly used alloying element in steel. The most important feature of the steel is that it adds a stainless feature to the steel thanks to the bright oxide layer it forms on the surface of the steel. There is approximately 12% chromium in the composition of stainless steel. Chrome also increases the hardness thanks to the carbides it creates in the steel. While it increases the tensile strength and heat resistance like carbon, it also decreases the ductility.
Nickel may be present in steel materials up to 5% by weight of the composition. Nickel improves the hardness and strength properties of the material without decreasing the ductility and toughness, unlike chromium and carbon. It is widely used in stainless steel.
Manganese improves the mechanical properties of steels. It increases the strength and decreases ductility. It increases the malleability by reacting with the sulfur contained in the composition. When viewed from a thermal point of view, it increases the quenching depth. Manganese’s ability to increase hardness and strength also depends on the carbon composition of the material. It may also cause an increase in the weldability of the material.
Sulfur is an undesirable alloying element other than free-cutting steel. Because it makes the steel brittle. For this reason, the effect is minimized by reacting with manganese. It is desired to be in composition as it facilitates machining in free-cutting steels.
Silicium is used as an oxygen and degassing agent during production. It also provides fluidity in casting. It improves the magnetic properties of steel and increases its heat resistance. While it increases the hardenability and wears resistance of the material, it adversely affects the surface quality.
Molybdenum: It is used to prevent temper brittleness in steels containing molybdenum, low chromium, and nickel. It increases the heat resistance of steel. Molybdenum has the effect of increasing the effects of other alloying elements. For this reason, it is popular to be used not alone, but with other alloying elements. Molybdenum combines with carbon to form carbide. Since carbides increase the hardness, it is common to use tool steels.
Vanadium; increases the strength, hardness, and wear resistance of steels. Small amounts of added vanadium can prevent grain coarsening. Tempering and softening processes after heat treatment do not give any results. For this reason, it is widely used in tool steels.
Tungsten; increases the wear resistance, hardness, and toughness of steels. It provides hot working and cutting efficiency to the material at high temperatures. For this reason, it is popular in tool steels and high-speed steels. It is preferred to use in the structure of heat-resistant steels.
Cobalt slows the grain coarsening at high temperatures. Increases the heat resistance of the material and strength at high temperatures. For this reason, it is preferred in tool steels.
Aluminum is used as a deoxidizer. It has a grain refinement feature, therefore it prevents the growth of austenite grains. Increases aging resistance. For this reason, deep-drawn sheets contain aluminum in their structure.
Phosphorus, like sulfur, turns steel into brittle. For this reason, phosphorus is also undesirable. It increases the hardenability of the steel. But it causes a huge drop in ductility. This decrease is observed more in high carbon steels.
Copper imparts corrosion resistance and hardness properties to steel. But at the same time, it decreases ductility very much. For this reason, it is kept at a maximum of 0.5% in the composition.
Nitrogen increases its strength and hardness properties. It increases the hardness by forming nitride in the structure of the steel. It facilitates the machining process. It increases fragility.
Reference


